A US perspective on the current and future
regulation of anticoccidial drugs and vaccines
Paul Duquette, Phibro Animal Health (paul.duquette@pahc.com)
65 Challenger Road, Third Floor
Ridgefield Park, New Jersey 07660 - USA
Abstract
The US broiler industry is currently thriving, with more than 8.5 billion
broilers produced per year. This success is partly due to the availability of
multiple anticoccidial drugs, both chemicals and polyether ionophores, and
anticoccidial vaccines. In the US, the Center for Veterinary Medicine (CVM),
Food and Drug Administration, is responsible for approval of new anticoccidial
drugs, while the United States Department of Agriculture (USDA) is responsible
for approval of anticoccidial vaccines. The CVM anticoccidial drug approval
process is quite rigorous, requiring demonstration of manufacturing capability,
efficacy, target animal safety, human food safety, and environmental safety. The
USDA approval process for anticoccidial vaccines, which differs somewhat from
the CVM approval process, also requires demonstration of manufacturing
capability, efficacy and safety. Although many anticoccidial drugs are currently
available (and expected to remain available), few, if any, new drugs are under
development, due to the high cost and length of the approval process. Only a few
anticoccidial vaccines are currently available, but new vaccines are under
development. The US and worldwide broiler industry should continue to thrive as
long as currently used anticoccidial drugs are used prudently, anticoccidial
vaccines are used when and where effective, and necrotic enteritis and other
diseases that exacerbate coccidiosis are controlled.
Introduction
The broiler industry in the US is currently a thriving industry. US broiler
producers currently produce more than of 8.5 billion broilers per year. The
industry relies on various feed additives, including growth promotants used to
enhance productivity and maintain the health status of the animals, therapeutic
antibiotics used for disease treatment, and anticoccidials. Without these
products, all of which are approved by FDA after extensive testing to
demonstrate efficacy and safety, it would be impossible for the industry to
maintain the quality of its products and to survive in its current form.
In order to understand the current and future of the industry in general, and
anticoccidials in particular, it is important to have a historical perspective.
History of the
US broiler industry and anticoccidials
In his campaign for the presidency in 1928, Herbert Hoover used a campaign
slogan “a chicken in every pot and a car in every garage”. Hoover’s intent
was to promise affluence to the US public. However, his slogan gives an
important perspective regarding the broiler industry. In 1928, only the
affluent, or farmers who grew their own, could afford to eat chicken, which was
considered a “high priced” protein source. Although numbers for broiler
production aren’t available for 1928, they are available beginning in 1940. In
1940, 143 million broilers were raised for consumption in the US. This number
grew to 631 million in 1950, 1.8 billion in 1960, 3 billion in 1970, 4 billion
in 1980, 5.8 billion in 1990 and 8.3 billion in 2000, and reached over 8.8
billion in 2004. During this time period, average live weight of broilers
increased from 3 pounds to over 5 pounds, feed conversion decreased from 4
pounds of feed per pound of broiler to under 2 pounds, mortality decreased from
10% to 5%, and market age decreased from 12 to less than 7 weeks. [Note that the
production data listed above was obtained from various United States Department
of Agriculture (USDA) publications. For more information on the US poultry
industry, see the USDA web site at http://www.ams.usda.gov.]
The tremendous improvements in the broiler industry can be attributed to many
factors. Intensive breeding programs have resulted in highly improved genetics.
Management practices include knowledge of nutrient requirements and improved
feedstuffs. Antibiotic feed additives, especially those that control necrotic
enteritis, allow for intensive rearing conditions with reduced mortality, while
growth promotants improve feed efficiency and growth rate.
Of course, without anticoccidial drugs and vaccines, the growth of the US
broiler industry described above would have been impossible, as coccidia are
ubiquitous and extremely deleterious to the growth and survival of broilers.
Fortunately, introduction of the sulfur/sulfonimide drugs in the 1930s started
an era of treating and preventing coccidiosis. Since that time, the emphasis has
been on prevention, with the introduction of the chemical anticoccidial drugs in
the 1940s through 1970s, introduction of the first polyether ionophores in the
1970s, and the more recent introduction of anticoccidial vaccines (although
vaccines have been used in broiler breeders since the 1950s, formulations for
broiler production are relatively new). Growth of the US broiler industry
demonstrates a high correlation with the discovery of new anticoccidials.
The current status of anticoccidials and anticoccidial regulation in the US
Anticoccidial drugs and vaccines are regulated by two US agencies. The Center
for Veterinary Medicine (CVM), Food and Drug Administration (FDA), regulates
anticoccidial drugs, while the United States Department of Agriculure (USDA)
regulates anticoccidial vaccines. As such, they will be discussed separately,
below.
Anticoccidial drugs
The US Food, Drug and Cosmetic Act mandates that a new animal drug may not be
sold in interstate commerce unless it is the subject of a New Animal Drug
Application (NADA). In order to obtain an NADA, a drug product sponsor must
demonstrate that the drug is safe and effective, and can be manufactured in a
manner that preserves its identity, strength, purity and quality. The Center for
Veterinary Medicine (CVM), Food and Drug Administration (FDA) is responsible for
the review and approval of NADAs. See U.S. Code: Title 21-Food and Drugs Part
514 – New Animal Drug Applications at
http://www.access.gpo.gov/nara/cfr/waisidx_98/21cfr514_98.html for more
information on the NADA process.
Twenty anticoccidial drugs/drug combinations are currently codified (approved)
for use in broilers in the US. Table 1 lists the approved US anticoccidial
drugs, their approved withdrawal time, and their tissue tolerances/safe
concentrations.
Table 1. Anticoccidial drugs approved for use in broilers in the US.a
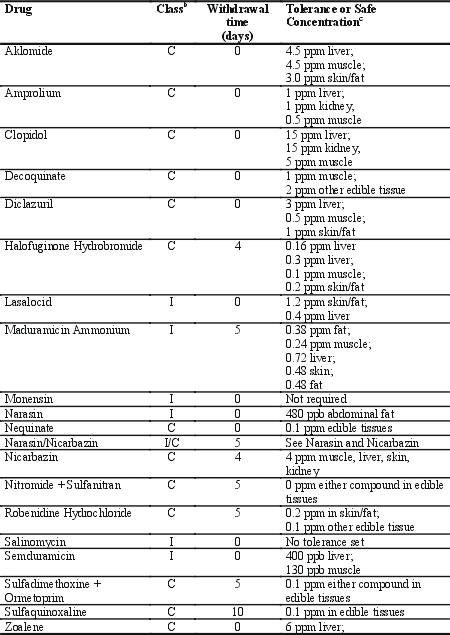
A Data from United States Code of Federal
regulations, 21 CFR Parts 556 (tolerances) and 558 (approvals and withdrawal
times), 2005. For further information, see
http://www.gpoaccess.gov/cfr/index.html.
b C = chemical; I = polyether ionophore.
c US FDA sets tissue tolerances and/or safe concentrations, which may differ
from European MRLs. Tolerance refers to a concentration of a marker residue in
the target tissue selected to monitor for total residues of the drug in the
target animal, and safe concentrations refers to the concentrations of total
residues considered safe in edible tissues. Tolerances are shown in normal font,
while safe concentrations are shown in italics. In cases where no safe
concentration is listed separately, the tolerance is the safe concentration.
Although all of the drugs listed in
Table 1 are approved, many of them are no longer marketed.
The US CVM approval process for animal health drugs used in food animal species
(including anticoccidial drugs) is quite rigorous and comprehensive. Submissions
for approval can be phased (each “technical section” submitted separately)
or complete (all “technical sections” submitted simultaneously). For further
information on phased submissions, see Guidance for Industry (GFI) #132 located
on the CVM website at http://www.fda.gov/cvm/Documents/dguide132.doc]; for
further information on complete submissions, see GFI #41
http://www.fda.gov/cvm/Guidance/Guideline41.htm). Whichever submission format is
chosen, the following technical sections are required:
· Chemistry, Manufacturing, and Controls
· Effectiveness
· Target Animal Safety
· Human Food Safety
· Environmental Impact
· Labeling
· Freedom of Information Summary
· “All other information”
Each of these sections will be discussed briefly.
The Chemistry, Manufacturing, and
Controls (CMC) section should contain complete information regarding the
manufacture of the new animal drug active ingredient and the new animal drug
product. It should contain information on personnel, facilities, components and
composition, manufacturing procedures, analytical specifications and methods,
control procedures, stability, containers and closures, Good Manufacturing
Practice (GMP) compliance, and other aspects of the chemistry and manufacturing
processes. CVM has published multiple GFIs that describe different aspects of
the CMC section process, including those listed in Table 2.
Table 2. CVM Guidance on Chemistry, Manufacturing and Controls.a
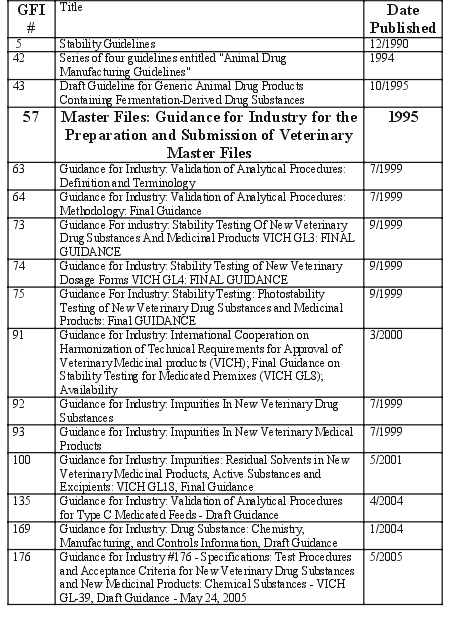
a All CVM GFI can be found on the CVM
website at
http://www.fda.gov/cvm/guidance/published.htm.
The Effectiveness section must contain full reports
of all studies that show whether or not the new animal drug is effective for its
intended use. CVM has published Guidance for Industry (GFI) that specifically
outlines the studies necessary to demonstrate effectiveness of anticoccidial
drugs. Specific studies described in this GFI include battery studies (with both
single Eimeria species and mixed cultures), floor pen challenge studies, and
field trials. For further information, see GFI #40, at:
http://www.fda.gov/cvm/Guidance/dguide40.pdf.
The Target Animal Safety technical section must contain full reports of all
studies that show whether or not the new animal drug is safe to the target
species. In addition, any target animal safety issues that become apparent in
efficacy studies must be reported in this section. Target animal safety study
design for poultry anticoccidial drugs is specifically mentioned in section IX
of GFI # 33, located at:
http://www.fda.gov/cvm/Guidance/Guideline33.htm.
The Human Food Safety technical section is the most comprehensive technical
section, and must contain information on residue toxicology, residue chemistry,
residue analytical methods, pharmacokinetics and bioavailability. CVM has
recently (June 2005) updated GFI #3, General Principles for Evaluating the
Safety of Compounds Used in Food-Producing Animals, including references to the
following Guidelines: Guidance for Metabolism Studies and for Selection of
Residues for Toxicological Testing; Guidance for Toxicological Testing; Guidance
for Establishing a Safe Concentration; Guidance for Approval of a Method of
Analysis for Residues; Guidance for Establishing a Withdrawal Period; Guidance
for New Animal Drugs and Food Additives Derived from a Fermentation; and
Guidance for the Human Food Safety Evaluation of Bound Residues Derived from
Carcinogenic New Animal Drugs. In addition, CVM has adopted various guidelines
(GL) developed by the International Cooperation on Harmonisation of Technical
Requirements for Registration of Veterinary Medicinal Products (VICH) regarding
safety studies for residues, including those listed in Table 3.
Table 3. VICH safety study guidelines adopted by CVM.a
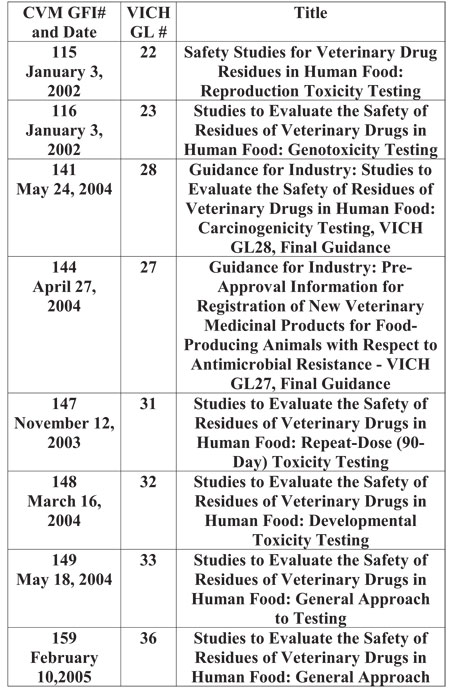
a These guidelines are available on the CVM
http://www.fda.gov/cvm/guidance/published.htm.
It should be noted that, for anticoccidial compounds
that have anti-infective properties (such as the polyether ionophores), CVM has
recently begun requiring studies on microbiology, including the effects of
residues on human intestinal microflora (GFI #159) and evaluation of the safety
with regard to their microbiological effects on bacteria of human health concern
antimicrobial resistance (GFI #152). GFI #152, which describes a
“qualitative” approach to antibacterial resistance risk assessment, may be
superceded by a more “quantitative” approach. Although no examples of a
quantitative risk assessment are available for the polyether ionophores, CVM has
published a draft risk assessment on virginiamycin, a product used in poultry to
control necrotic enteritis and improve growth rate and feed efficiency. The CVM
document:
(http://www.fda.gov/cvm/Documents/SREF_RA_FinalDraft.pdf) demonstrates the CVM
approach to risk assessment (and also demonstrates that the continued use of
virginiamycin poses no significant risk to human health).
The Environmental Impact section must, by regulation, contain either an
environmental assessment (EA), or a request for categorical exclusion. A claim
of categorical exclusion must include a statement of compliance with the
categorical exclusion criteria and must state that to the sponsor’s knowledge,
no extraordinary circumstances exist. “Environmental Impact Considerations”
and directions for preparing an EA can be found in 21 CFR Part 25. In addition,
CVM has adopted 2 VICH environmental guidelines, GL6 [CVM GFI #89 Guidance for
Industry - Environmental Impact Assessments (EIA's) For Veterinary Medicinal
Products (VMP's) - Phase I, http://www.fda.gov/cvm/Guidance/guide89.doc] and
GL38 [GFI #166 Draft Guidance for Industry - Environmental Impact Assessments
(EIA's) for Veterinary Medicinal Products (VMP's) - Phase II:
http://www.fda.gov/cvm/Guidance/dguide166.doc].
The Labeling section should include facsimile copies of container labels,
package inserts and any other labeling that will be used with the products. For
medicated feeds, copies of representative labeling for the Type B and Type C
medicated feeds, referred to as “Blue Bird” labeling, should also be
included. Facsimile labeling is nearly final labeling that adequately reproduces
the package size (actual or to scale); graphics; pictures; type size, font, and
color of text; and the substance of the text to demonstrate to the reviewing
Division that the final printed labeling will be in compliance with applicable
regulations. Labeling should address any user safety concerns identified during
the review process. CVM has not published final guidance on labeling; however,
labeling requirements are codified in 21 CFR Part 201:
(http://www.access.gpo.gov/nara/cfr/waisidx_98/21cfr201_98.html).
The completed Freedom of Information Summary (FOI) Summary should include the
specific language relevant to a technical section that was agreed upon during
the review of the individual technical section (e.g., the tolerance and
withdrawal time for a new animal drug intended for use in food-producing
animals) and should be accepted by the Division responsible for the evaluation
of the target animal safety technical section. For further information on FOI
summaries, see GFI # 16 (FOI Summary Guideline,
http://www.fda.gov/cvm/Guidance/Guideline16.htm).
The All Other Information section must
include all other information, not included in any of the other technical
sections, that is pertinent to an evaluation of the safety or effectiveness of
the new animal drug for which approval is sought. All other information
includes, but is not limited to, any information derived from other marketing
(domestic or foreign) and favorable and unfavorable reports in the scientific
literature.
It should be noted that, in the US, anticoccidial drugs cannot be used in
combination with any other drugs until the combination is approved by CVM (see
CVM Guidelines for Drug Combinations for Use in Animals, GFI # 24, at
http://www.fda.gov/cvm/Guidance/Guideline24.htm.
For further information on the animal drug approval process in the US, visit the
CVM website and associated links at:
http://www.fda.gov/cvm/nadaappr.htm.
Anticoccidial
vaccines
The U.S. Department of Agriculture (USDA) is authorized, under the 1913
Virus-Serum-Toxin Act (see http://www.aphis.usda.gov/vs/cvb/vsta.htm) as amended
by the 1985 Food Security Act, to ensure that all veterinary biologics produced
in, or imported into, the United States are not worthless, contaminated,
dangerous, or harmful. Federal law prohibits the shipment of veterinary
biologics unless they are manufactured in compliance with regulations contained
in Title 9 of the Code of Federal Regulations, Parts 101 to 118. Veterinary
biologics for commercial use must be produced at a USDA-approved establishment,
and be demonstrated to be pure, safe, potent, and efficacious.
Three anticoccidial vaccines (Table 4) are currently USDA approved for use in
broilers in the US.
Table 4. Anticoccidial vaccines
approved for use in broilers in the US.a
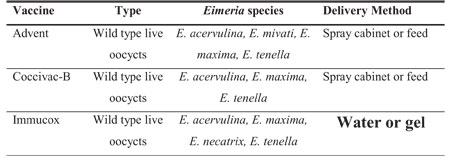
Note in the above table that all currently approved
vaccines contain wild type live oocysts, but vary in the Eimeria species they
contain and their delivery method.
The USDA vaccine approval process is outlined as follows (see
http://www.aphis.usda.gov/vs/cvb/lpd/lpdfaqs.htm):
In order to manufacture and sell veterinary biologics in the US, domestic
manufacturers are required to possess a valid U.S. Veterinary Biologics
Establishment License and an individual U.S. Veterinary Biologics Product
License for each product produced for sale.
Foreign manufacturers of veterinary biologics may export veterinary biologics to
the United States, provided that the manufacturer's legal representative
(permittee) residing in the United States possesses a valid U.S. Veterinary
Biological Product Permit to import these products for general distribution and
sale.
The following documentation must be submitted to USDA for a biologics
establishment license:
· Application for United States Veterinary Biologics Establishment License
· Articles of Incorporation for
applicant and any subsidiaries if a corporation
· Water quality statement
· An application for at least one United States Veterinary Biological Product
License
· Personnel Qualifications Statement for supervisory personnel
· Blueprints, plot plans, and legends
The following documentation must be
submitted to USDA for a biologics product license:
· Application for a United States Veterinary Biological Product License
· Outline of Production and, if applicable, Special Outlines
· Master Seed data, including test results for purity, safety, identity, and
genetic characterization
· Master Cell Stock data, including test results for purity and identity
· Product safety data from laboratory animal and contained host animal studies
· Host animal immunogenicity/efficacy data
· Field safety data
· Labels for all containers, cartons, and enclosures (circulars)
· Potency test validation data, including correlation with host animal efficacy
3 consecutively produced pre-license serials that have tested satisfactorily for
purity, safety, and potency
Guidelines (Veterinary Services
Memorandums) on preparation and submission of the above information can be found
at the USDA website, http://www.aphis.usda.gov/vs/cvb/vsmemos.htm.
In addition to the approved vaccines listed in Table 2, vaccines may be produced
and used without USDA approval, provided that:
· The product was manufactured by veterinarians and intended solely for use
with their clients' animals under a veterinarian-client-patient relationship.
· The product was manufactured by individuals or companies for use only in
their own animals.
· The product was manufactured in States with USDA-approved veterinary
biologics regulatory programs, for sale only in those States.
The future of
anticoccidials and anticoccidial regulation in the US
The Animal Health Institute (AHI) has recently published a news release entitled
“Antibiotic Use in Animals Rises in 2004” (see
http://www.ahi.org/mediaCenter/documents/Antibioticuse2004.pdf) that shows that
ionophore/arsenical (grouped to abide by disclosure agreements) sales in the US
increased from 8,644,638 pounds in 2003 to 9,444,107 pounds in 2004. At first
glance, this appears to be good news for the animal health industry; however,
these data demonstrate increased reliance on existing compounds, as no new
compounds have been approved in the past few years. In fact, the last new
anticoccidial drug approval in the US occurred in 1999, when diclazuril was
approved. Prior to diclazuril, semduramicin was approved in 1994. The approval
of new anticoccidial drugs has come to a standstill due to the high cost and
length of time it takes for approval with the current approval requirements:
estimates are that it currently costs more than $25 million and at least 10
years to obtain CVM approval of a new anticoccidial drug (information based on
AHI sponsor input). Industry information suggests that no new anticoccidial
drugs are under development. The good news is that currently marketed
anticoccidial drugs will remain on the market, as there is no indication that
CVM has any concerns with these compounds.
If no new anticoccidial drugs will become available, what will happen to the US
and worldwide broiler industry? It appears that new anticoccidial vaccine
technology may fill some of the void. Dalloul and Lillehoj (2005) have recently
published a paper describing functional genomic technology and recent advances
in live and recombinant vaccine development that may result in broiler
vaccination strategies that work better than current vaccines. Bedrnik (2004)
reviews information on multiple anticoccidial vaccines that have been or are
being developed worldwide. MacDougald et al. (2005) have recently patented
vaccine for coccidiosis in chickens prepared from four attenuated Eimeria
species: E. acervulina, E. maxima, E. mitis and E. tenella (see
http://www.uspto.gov/ patent number 6,908,620). Industry sources suggest that at
least 4 new anticoccidial vaccines are currently under development for the US
market.
However, anticoccidial vaccines are not the complete answer as they are not
always effective and do nothing to control necrotic enteritis, and anticoccidial
drugs must continue to be used. The broiler industry must use these products
prudently to avoid over use and possible resistance issues. Shuttle programs
must be adopted to “rest” popular anticoccidial drugs. Both chemical and
polyether ionophore anticoccidial drugs should be used as seasonal conditions
and coccidial challenge allow. Efforts should be made to control necrotic
enteritis and other disease conditions that may exacerbate coccidiosis.
The US and worldwide broiler industry should continue to thrive as long as
currently used anticoccidial drugs are used prudently, anticoccidial vaccines
are used when and where effective, and necrotic enteritis and other diseases
that exacerbate coccidiosis are controlled. The anticoccidial drugs are amongst
the most important tools that allow the broiler producer to produce healthy
birds, because they are the safest and most effective method to control
coccidiosis. In turn, the consumer benefits through low-priced, high-quality
animal protein produced using drugs that have been proven safe and effective in
rigorous scientific studies.
References
[Note: much of the information presented in this paper was gathered from
official US government websites, which have been referenced throughout this
document. Key websites used, and referenced publications, are listed below.]
Websites
United States Department of Agriculture, Agriculture Marketing Service at
http://www.ams.usda.gov
United States Code of Federal
regulations, 21 CFR Parts 556 (tolerances) and 558 (approvals and withdrawal
times), 2005 at:
http://www.gpoaccess.gov/cfr/index.html
United States Food and Drug
Administration, Center for Veterinary Medicine guidance documents at
http://www.fda.gov/cvm/guidance/published.htm
United States Department of Agriculture,
Center for Veterinary Biologics, Veterinary Services Memorandums (guidance
documents) at:
http://www.aphis.usda.gov/vs/cvb/vsmemos.htm
United States Patent and Trademark
Office at http://www.uspto.gov/
Animal Health Institute at
http://www.ahi.org/
Publications
Bedrnik, P., 2004. Control of Poultry Coccidiosis in
the 21st Century. Praxis veterinaria 52 (1-2):49 – 54.
Dalloul, R.A. and H.S. Lillehoj,
2005. Recent Advances in Immunemodulation and Vaccination Strategies Against
Coccidiosis. Avian Diseases 49:1-8.



